Ampia Gamma e Qualità Certificata
Microtrace, Salute sotto Controllo
Il nostro lavoro ci spinge ogni giorno a cercare, ideare e mettere in pratica soluzioni efficaci per avvicinare sempre di più salute e paziente.
Come?
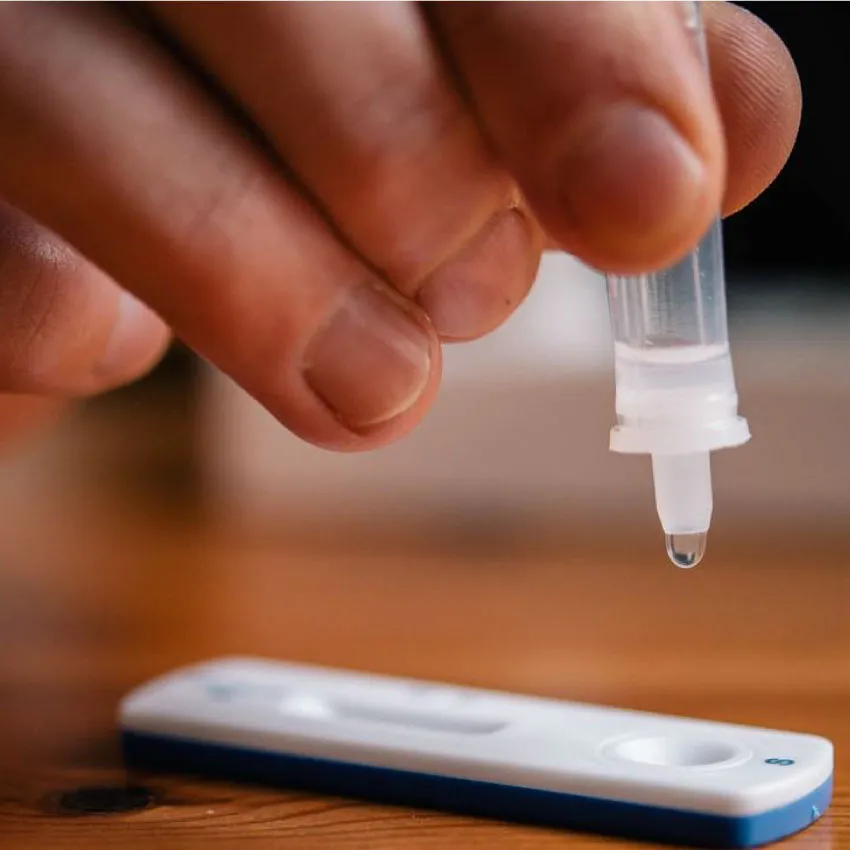
Mettendo a disposizione di tutti il nostro laboratorio analisi e test diagnostici affidabili, facili e veloci da eseguire.





![[DUPLICATO|15] [DUPLICATO|1] Test intolleranza alimentare Metodo [DUPLICATO|15] [DUPLICATO|1] Test intolleranza alimentare Metodo](/upload/prodotti/20240422102318_1_allergie-alimentari-|-inalanti.webp)
![[DUPLICATO|1] Test intolleranza alimentare Metodo [DUPLICATO|1] Test intolleranza alimentare Metodo](/upload/prodotti/20240422102232_1_test-intolleranze-alimentari.webp)
![[DUPLICATO|15] [DUPLICATO|1] Test intolleranza alimentare Metodo [DUPLICATO|15] [DUPLICATO|1] Test intolleranza alimentare Metodo](/upload/prodotti/20240422102423_1_microbiota-intestinale.webp)












